Seaweed extract, as an efficient plant biostimulant[1], has its biological activity and field performance highly dependent on the production process. Different extraction technologies directly influence the retention of active compounds, product stability, and final efficacy in agricultural practice. From traditional physical and chemical extraction to modern enzymatic extraction, the evolution reflects not only technological innovation but also the industry's response to the needs of green agriculture and sustainable development. Through its independently developed compound-enzyme directed enzymolysis technology, Qingdao Seawin Biotech Group Co., Ltd. has successfully over come industry bottlenecks and redefined both the efficiency and sustainability of kelp (Laminaria Japonica) active-ingredient extraction.

Traditional Physical Extraction: High Energy Consumption and Limited Active-Compound Retention
High-Temperature Water Extraction
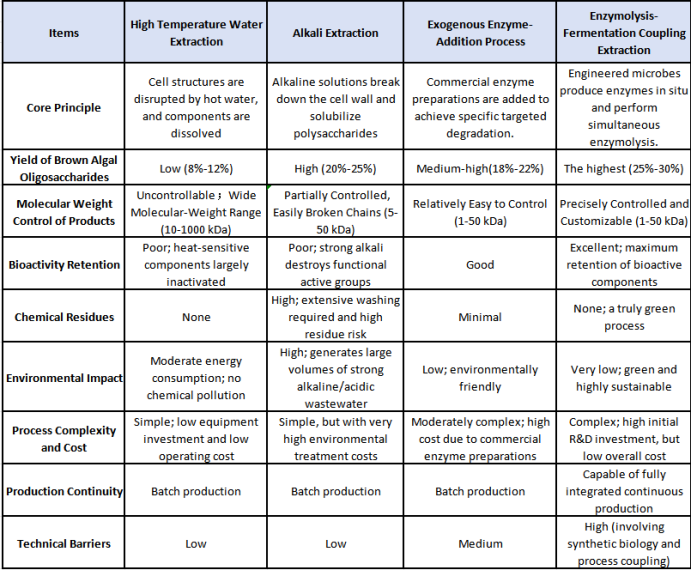
This classical extraction method relies on high-temperature aqueous immersion after kelp is mechanically crushed. It enables the recovery of heat-stable components such as alginic acid[1], and features simple equipment requirements and relatively low cost. However, temperatures above 80 °C cause substantial degradation of heat-sensitive substances (e.g., endogenous auxins, fucoidan), resulting in an active-compound retention rate typically below 50%. The extracted material shows a very broad molecular-weight distribution (10–1000 kDa), making it difficult for plants to absorb efficiently. Data indicate that the alginic-acid yield from this process is usually only 8–12%, which falls short of the requirements for producing high-activity biostimulants.
Mechanical Disruption Method
Using physical forces such as ball milling or high-pressure homogenization to disrupt kelp cell walls avoids chemical-reagent contamination. However, due to the unique cellulose–alginic acid composite structure of kelp cells, the wall-breaking efficiency remains low. Energy consumption is significant (approximately 12–15 kW·h/ton of material processed), and the polysaccharide release rate is generally below 15%, which limits the scalability of this method.
Chemical Extraction: Balancing Higher Efficiency with Environmental Concerns
Alkali Extraction Method
Using alkaline solutions such as potassium hydroxide or sodium carbonate at 60–80 °C can increase the extraction rate of alginic acid to 20–25%. However, the strong alkaline environment (pH > 10) easily causes hydrolysis of the sulfate groups in fucoidan and deactivation of natural plant hormones. At the same time, processing each ton of raw kelp generates approximately 3–5 tons of alkaline wastewater, leading to high treatment costs and significant environmental impact.
Acid Extraction Method
Treatment with dilute sulfuric acid or other acids can effectively extract iodine and mineral elements. However, the acidic conditions (pH < 3) cause polysaccharide chain cleavage, reducing the molecular weight to below 5 kDa, and also lead to protein denaturation and Maillard browning reactions, which negatively affect product stability and appearance.
Although optimizing reaction temperature and duration can mitigate some degradation, concerns over chemical residues continue to limit the use of this method in organic agriculture. At the same time, the environmental impact associated with chemical processing is drawing increasing attention across the industry.
Enzymolysis Extraction Method[1]: The Core Pathway of Eco-Friendly Biomanufacturing
Enzymolysis technology[1] uses the specific catalytic activity of enzymes to efficiently degrade kelp cell walls and polysaccharide components under mild conditions, maximizing the retention of bioactive compounds. It has become the mainstream development direction for kelp-based extraction processes. Depending on the enzyme source and processing route, enzymatic extraction can be classified into traditional exogenous enzyme-addition methods and the new generation of fermentation–enzymolysis coupling technologies. Leveraging synthetic biology, Qingdao Seawin Biotech Group has developed high-efficiency engineered microbial strains, enabling a fully integrated production system—from enzyme preparation to targeted enzymolysis of kelp polysaccharides—representing a leading level of innovation in this field.
Traditional Exogenous Enzyme-Addition Process
Traditional enzymolysis extraction relies on commercially available enzyme preparations, with the enzyme-production stage separated from the enzymatic hydrolysis step.
Enzyme sources: Direct procurement of commercial cellulases, alginate lyases, proteases, and other enzyme products.
Process characteristics: The kelp raw material must be pretreated (crushing, pH adjustment, temperature control), followed by the addition of exogenous enzymes. After the reaction, the enzymes are deactivated, and the mixture is then subjected to separation and purification.
Advantages: Relatively simple to operate, easy to implement, and avoids the high-temperature and strong-alkali conditions typical of chemical extraction.
Limitations:
a. The cost of enzyme preparations is high, accounting for approximately 30–40% of total production cost.
b. Variability in enzyme activity between different commercial batches affects product consistency and process stability.
c. The fixed composition of commercial enzyme mixtures makes it difficult to flexibly optimize the system for different kelp raw materials.
d. The enzymolysis efficiency and completeness are generally inferior to precisely controlled multi-enzyme synergistic systems.
SEAWIN Enzymolysis- Fermentation Coupling Technology
This process systematically integrates the enzyme-producing fermentation of engineered strains with the degradation and extraction of kelp polysaccharides, achieving full-chain synergy across “engineered strain fermentation[1] – substrate deconstruction – active-compound release.” It fundamentally transforms the traditional segmented enzymatic-hydrolysis production model. Its technological innovations are reflected in the following key stages: Core Concept: Integrated Microbial Cell Factory and Multi-Enzyme Synergy System Engineered strain development: Using synthetic biology tools, specific microorganisms (e.g., Bacillus subtilis) are genetically modified to construct engineered strains capable of efficiently secreting multiple enzyme systems, including cellulases, alginate lyases, fucoidanases, and proteases. Fermentation–enzymolysis coupling: Within a single reaction system, both enzyme biosynthesis and the targeted degradation of kelp polysaccharides occur simultaneously, breaking the traditional “enzyme production followed by enzymolysis” sequential model. Intelligent process control: Integrated online monitoring and feedback systems regulate key parameters such as temperature, pH, dissolved oxygen, and substrate concentration in real time during fermentation and enzymolysis, ensuring process stability and reproducibility.
Multi-Enzyme Synergy Mechanism:
Engineered strains, modified through synthetic biology, can simultaneously secrete cellulases, alginate lyases, fucoidanases, and other enzymes. By regulating the expression ratio of each enzyme through genetic engineering, a coordinated “enzyme attack network” is formed to target the complex structure of kelp cell walls and intracellular polysaccharides. This coordinated system significantly enhances the yield and purity of key bioactive components.
a. Cellulases first degrade the β-1,4-glucan backbone of the cell wall, releasing alginic acid and fucoidan.
b. Alginic acid lyases specifically cleave the β-1,4 linkages of alginate in the intercellular matrix, generating low-molecular-weight oligosaccharides.
c. Fucoidanases selectively cleave the glycosidic bonds of fucoidan while preserving the active sulfate groups (avoiding the desulfation observed in traditional acid/alkali extraction), thereby ensuring the biological activity of the final products.
Technical Advantages Comparison:
Development of Innovative Assisted Extraction Technologies
1. Ultrasonic-Assisted Extraction
Ultrasonic cavitation enhances cell disruption, significantly shortening extraction time and reducing operating temperature. Studies have shown that this method can increase fucoidan yield by more than 40% and reduce energy consumption by approximately 30%. However, equipment investment is relatively high, and the impact on product structure and molecular-weight distribution still requires systematic evaluation.
2. Supercritical CO₂ Extraction
This technology is suitable for high-purity extraction of lipophilic active compounds such as fucoxanthin and plant hormones. Under conditions of 31 °C and 7.4 MPa, the extraction purity can exceed 98%. However, due to its high operational cost, it is currently used mainly in the pharmaceutical sector and for producing high-value raw materials.
Outlook: Enzyme Engineering Shaping the Future
The extraction of bioactive compounds from kelp is gradually evolving from extensive, high-pollution processes toward refined, green, and sustainable technologies. With its clear advantages in activity retention and environmental compatibility, enzymolysis[1] has become the dominant pathway in the industry. Qingdao Seawin Biotech Group is actively advancing the development of thermostable alginate lyases and immobilized-enzyme reactors to further shorten reaction times and enable enzyme reuse. As breakthroughs in enzyme engineering—such as thermostable enzyme formulations and customized multi-enzyme systems—continue to emerge, and as multi-technology coupling (e.g., ultrasound–enzymatic hydrolysis–membrane separation) becomes more widely adopted, kelp extraction processes will become increasingly green, intelligent, and precise, providing a stronger technological foundation for sustainable agriculture. In this transformation, Chinese enterprises are leveraging technological innovation and full-value-chain integration to shift from followers to innovation leaders in the global biomanufacturing landscape.



